Table of Contents
Introduction
When the body goes through different situations, it has the natural ability to repair itself. Sometimes the injuries can be so severe that functional and holistic therapies can help alleviate any kind of pain that a person is feeling. It can range from having inflammation throughout the body or certain areas to damaged tissues that can cause mobility issues for a person. When this happens, there are many therapy options that can help the body alleviate the cause of these chronic issues and one of them is human cellular tissue products therapy or HCTP therapy. In this 2 part series, we will be taking a look at what is HCTP therapy and how it is different than other forms of therapy. In Part 2, we will be discussing more on what HCTP therapy is as well as the procedures to HCTP therapy. By referring patients to qualified and skilled providers that specialized in regenerative cellular therapy, we work with affiliated clinics and distributor organizations, both internationally and nationally with the services that we offer. We find that education is the key to asking valuable questions to our providers. Dr. Alex Jimenez DC provides this information as an educational service only. Disclaimer
Can my insurance cover it? Yes, in case you are uncertain here is the link to all the insurance providers we cover. If you have any questions, please call Dr. Jimenez at 915-850-0900.
What Is HCTP Therapy?
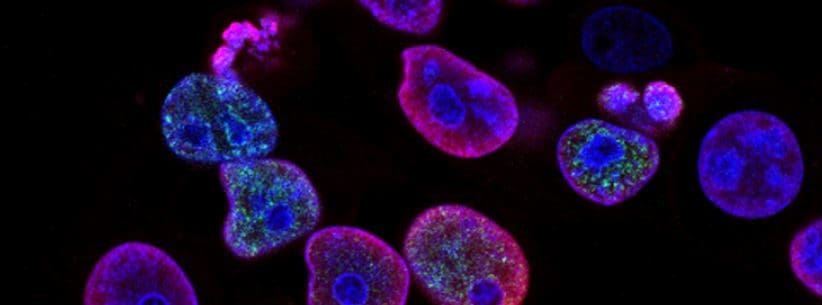
HCTP or human cellular tissue products are a form of regenerative medicine that can help the body’s recovery process immensely. Research has found that HCTP are unspecialized cells in the human body that can differentiate into any tissue cells and have the ability to self-renewal. HCTP therapy can help the body’s own natural repairing process by repairing and regenerating damaged body cells, tissues, and organs to their original function. Research studies have shown that HCTP therapies have become one of the promising options since it helps replace diseased cells in the body. When individuals are going into HCTP therapy, there are pre- and post procedures that they must follow in order to have beneficial results from HCTP. However, only affiliated clinics and distribution organizations (both internationally and nationally) are allowed to do HCTP therapies for individuals that need them.
How Is It Different?
The mission and purpose that affiliated international clinics are to provide patients from all around the world access to healthcare services that will improve the quality of their life. With this step-by-step guide on how to help bring first-time and repeat patients on a comfortable international trip to Colombia for HCTP therapy. So how does affiliated international clinics stand apart from the nationally affiliated clinics here in the U.S.? The difference is that international clinics only received HCTP from donors that are screened by their top chosen gynecologist, who specializes in screening donors. They do this to ensure that they are consistently and accurately receiving the best cells from the best donors. In Colombia, the HCTP therapy procedures are different in the United States since Colombia is legally allowed to do more than manipulate HCTP and have the opportunity to:
- Remove any detrimental material that would cause negative effects, and then be able to safely do an IV application.
- Let the HCTP do what they do best: proliferate (increase and multiply).
How Is HCTP Therapy Different Internationally?
Since universal standards require 80% of cell viability to be alive, international affiliated clinics actually reject anything under 90% cell viability to ensure that patients receive the best results. There is also a huge difference between HCTP therapies in the United States and internationally in Colombia. With the United States using HCTP therapy:
- Has to be FDA-approved
- MSC’s from Wharton’s Jelly
- MSC’s can not be expanded and only minimally manipulated
- Not approved for IV use federally in the US
- 2 million stem cells per CC
- Cryopreserved
- It costs $2,500 for every 1 million stem cells
Research shows that around 2001 the president put a ban on funding the research for HCTP therapy and some states have been permitted their limited use. HCTP therapies in nationally affiliated clinics were only allowed for bone marrow treatments using HCTP therapy. For international affiliated clinics in Colombia using HCTP therapy can:
- Is funded by Invima (Colombia’s version of the FDA)
- MSC’s from Wharton’s Jelly
- MSC’s can be expanded
- The stem cell is approved for IV application
- 1 hundred million stem cells per CC
- Not cryopreserved
- It costs $114 for every 1 million stem cell
What this does is that international affiliated clinics and distribution organizations can continue with researching HCTP and widen the scope for them to be used in regenerative medicine. This will allow more scientific discoveries for HCTP therapies being used to reduce inflammation in the body or even repair damaged joint cartilages.
The First Part Of HCTP Therapy
The requirements that HCTP donors must meet when being screened in international affiliated clinics is that they must be young and healthy and have their HCTP be young and healthy as well. They must also pass what the FDA requires and under § 1271.50(b), a donor is eligible only if the screening shows that the donor is free from risk factors for, and clinical evidence of, infection due to relevant communicable
disease agents and diseases, and is free from communicable disease risks associated with xenotransplantation. Not only that but their test results for relevant communicable disease agents are
negative or nonreactive, except as provided in § 1271.80(d)(1) for non-treponemal screening tests for syphilis. These include:
- Human T-lymphotropic virus (HTLV), types I and II
- Chlamydia trachomatis
- Neisseria gonorrhea
Also, many international affiliated clinics make sure to check their patients for any heredity diseases to ensure the safety of the individual.
The Second Part Of HCTP Therapy
Once the first set of screenings has been performed and passed, it is then explained to the donors that half of the umbilical cord HCTP cells will be preserved for them for future use, the other half thenceforth is used for the patients. A donation kit is given to the mother so that when she is giving birth, she has it readily available to give the doctor looking after her pregnancy. The doctor that will be responsible for the donated umbilical cord knows exactly what to do with the kit to meet the strict protocols. This is to make sure that the HCTP is isolated from the blood – and is the only thing that is gathered.
Since these umbilical HCTP cells are from a universal donor, anyone can receive treatment, with no negative side effects. Many distribution organizations work exclusively with one of two trusted labs for chromosome testing. Once the results have come back, only then do affiliated providers can move forward in the quality control process. For the last part, many affiliated clinics have their own genetics lab, where they actually do paternity tests and also make sure the DNA collected has 16 genes. Once it is cultivated, they make sure no mutation has occurred. Then and only then do they actually grow HCTP to be cultivated for 7 days to ensure that the HCTP doesn’t become contaminated. By constantly sending samples to be tested for guaranteed quality and effectiveness, the original HCTP is cultivated and has no discrepancies. It can be counted and verified so the affiliated clinics and international doctors have the minimum required HCTP count to be used for treatments and therapies.
Conclusion
All in all, HCTP therapy is beneficial to the body since it can help speed up the body’s healing process naturally and even help repair and regenerate damaged cellular structures back to their original state. When there are unwanted pathogens that affect the body by damaging the body’s cellular structure and causing chronic illnesses to rise over time, it can cause the individual pain and make them miserable. Individuals who need HCTP therapy can begin their healing process and continue on with their wellness journey.
References
Aly, Riham Mohamed. “Current State of Stem Cell-Based Therapies: An Overview.” Stem Cell Investigation, AME Publishing Company, 15 May 2020, https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7367472/.
Reisman, Miriam, and Katherine T Adams. “Stem Cell Therapy: A Look at Current Research, Regulations, and Remaining Hurdles.” P & T: a Peer-Reviewed Journal for Formulary Management, MediMedia USA, Inc., Dec. 2014, https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4264671/.
Zakrzewski, Wojciech, et al. “Stem Cells: Past, Present, and Future.” Stem Cell Research & Therapy, BioMed Central, 26 Feb. 2019, https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6390367/.
Disclaimer
General Disclaimer
Professional Scope of Practice *
The information herein on "A Guided Look Into HCTP Therapy | Part 1" is not intended to replace a one-on-one relationship with a qualified health care professional or licensed physician and is not medical advice. We encourage you to make healthcare decisions based on your research and partnership with a qualified healthcare professional.
Blog Information & Scope Discussions
Welcome to El Paso's Premier Wellness and Injury Care Clinic & Wellness Blog, where Dr. Alex Jimenez, DC, FNP-C, a Multi-State board-certified Family Practice Nurse Practitioner (FNP-BC) and Chiropractor (DC), presents insights on how our multidisciplinary team is dedicated to holistic healing and personalized care. Our practice aligns with evidence-based treatment protocols inspired by integrative medicine principles, similar to those found on this site and our family practice-based chiromed.com site, focusing on restoring health naturally for patients of all ages.
Our areas of multidisciplinary practice include Wellness & Nutrition, Chronic Pain, Personal Injury, Auto Accident Care, Work Injuries, Back Injury, Low Back Pain, Neck Pain, Migraine Headaches, Sports Injuries, Severe Sciatica, Scoliosis, Complex Herniated Discs, Fibromyalgia, Chronic Pain, Complex Injuries, Stress Management, Functional Medicine Treatments, and in-scope care protocols.
Our information scope is multidisciplinary, focusing on musculoskeletal and physical medicine, wellness, contributing etiological viscerosomatic disturbances within clinical presentations, associated somato-visceral reflex clinical dynamics, subluxation complexes, sensitive health issues, and functional medicine articles, topics, and discussions.
We provide and present clinical collaboration with specialists from various disciplines. Each specialist is governed by their professional scope of practice and their jurisdiction of licensure. We use functional health & wellness protocols to treat and support care for musculoskeletal injuries or disorders.
Our videos, posts, topics, and insights address clinical matters and issues that are directly or indirectly related to our clinical scope of practice.
Our office has made a reasonable effort to provide supportive citations and has identified relevant research studies that support our posts. We provide copies of supporting research studies upon request to regulatory boards and the public.
We understand that we cover matters that require an additional explanation of how they may assist in a particular care plan or treatment protocol; therefore, to discuss the subject matter above further, please feel free to ask Dr. Alex Jimenez, DC, APRN, FNP-BC, or contact us at 915-850-0900.
We are here to help you and your family.
Blessings
Dr. Alex Jimenez DC, MSACP, APRN, FNP-BC*, CCST, IFMCP, CFMP, ATN
email: coach@elpasofunctionalmedicine.com
Multidisciplinary Licensing & Board Certifications:
Licensed as a Doctor of Chiropractic (DC) in Texas & New Mexico*
Texas DC License #: TX5807, Verified: TX5807
New Mexico DC License #: NM-DC2182, Verified: NM-DC2182
Multi-State Advanced Practice Registered Nurse (APRN*) in Texas & Multistate
Multistate Compact RN License by Endorsement (42 States)
Texas APRN License #: 1191402, Verified: 1191402 *
Florida APRN License #: 11043890, Verified: APRN11043890 *
* Prescriptive Authority Authorized
ANCC FNP-BC: Board Certified Nurse Practitioner*
Compact Status: Multi-State License: Authorized to Practice in 40 States*
Graduate with Honors: ICHS: MSN-FNP (Family Nurse Practitioner Program)
Degree Granted. Master's in Family Practice MSN Diploma (Cum Laude)
Dr. Alex Jimenez, DC, APRN, FNP-BC*, CFMP, IFMCP, ATN, CCST
My Digital Business Card
RN: Registered Nurse
APRNP: Advanced Practice Registered Nurse
FNP: Family Practice Specialization
DC: Doctor of Chiropractic
CFMP: Certified Functional Medicine Provider
MSN-FNP: Master of Science in Family Practice Medicine
MSACP: Master of Science in Advanced Clinical Practice
IFMCP: Institute of Functional Medicine
CCST: Certified Chiropractic Spinal Trauma
ATN: Advanced Translational Neutrogenomics